NEWS RELEASE 28-JUL-2023
Total recall on HIV
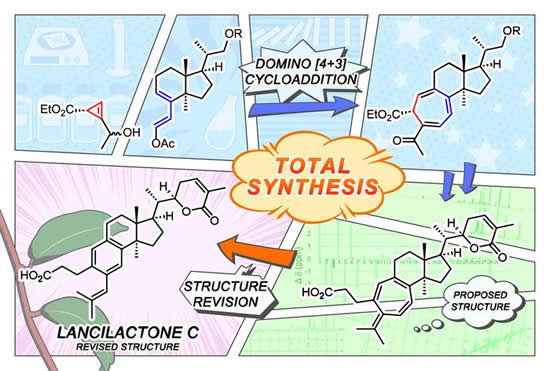
CAPTION: Flow of structure revision and synthesis of lancilactone C.
CREDIT: KyotoU/ Ayumi Uchino
Kyoto, Japan — Having control over how a dish is cooked is always a good idea. Taking a hint from the kitchen, scientists appear to have discovered a way to produce a true structure of the rare but naturally-occurring anti-HIV compound Lancilactone C from start to finish.
Its non-cytotoxicity in mammals could make this triterpenoid an ideal candidate for treating AIDS if its biological activity were clear -- and if only it were abundant in nature.
Now, a research group at Kyoto University has succeeded in creating a domino-like synthesis of Lancilactone C's unique seven-membered ring structure.
"Our synthetic method revealed that the proposed structure of Lancilactone C was initially incorrect," says Chihiro Tsukano of Kyoto University's Graduate School of Agriculture. "But we successfully derived its true structure from our spectral data and understanding of its biosynthesis."
In addition to this revelation, Tsukano realized that the electrocyclization -- a rearrangement reaction in organic chemistry -- used in the total synthesis also occurs in biosynthesis. Ironically, it remains a mystery of whether the proposed structure containing an unsaturated seven-membered ring might exist in nature as an analog -- or equivalent compound -- and how it might affect the expression of biological activity.
Tsukano's team utilized the domino-like reaction to enable the total synthesis of lancilactones and related triterpenoids. This outcome has inspired the team to further their research in optimizing compound structures, leading to possible development of novel antivirals.
The endless loop of required medication and multi-drug therapies often correlates with a lower quality of life for economically burdened patients.
"Our synthetic method for Lancilactone C, with its known efficacy, may lead to developing less problematic anti-HIV drugs," concludes Tsukano.
###
The paper "Total Synthesis and Structure Revision of (+)-Lancilactone C" appeared on 16 June 2023 in the Journal of the American Chemical Society, with doi: 10.1021/jacs.3c04124
About Kyoto University
Kyoto University is one of Japan and Asia's premier research institutions, founded in 1897 and responsible for producing numerous Nobel laureates and winners of other prestigious international prizes. A broad curriculum across the arts and sciences at undergraduate and graduate levels complements several research centers, facilities, and offices around Japan and the world. For more information, please see: http://www.kyoto-u.ac.jp/en
JOURNAL
Journal of the American Chemical Society
DOI
10.1021/jacs.3c04124
METHOD OF RESEARCH
Experimental study
SUBJECT OF RESEARCH
Not applicable
ARTICLE TITLE
Total Synthesis and Structure Revision of (+)-Lancilactone C
ARTICLE PUBLICATION DATE
16-Jun-2023
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.
Media Contact
Jake G. Tobiyama
Kyoto University
tobiyama.gakuji.6y@kyoto-u.ac.jp
Source: https://www.eurekalert.org/news-releases/997050
"Reproduced with permission - Kyoto University"
Kyoto University
For more HIV and AIDS News visit...
Positively Positive - Living with HIV/AIDS:
HIV/AIDS News
|