Targeting the Integrator complex improves reactivation of HIV
From the Emerman Lab, Human Biology Division
May 26 2025 | By H Lewis
It is not an exaggeration to say that the development of antiretroviral therapy is one of the most significant accomplishments in the history of medicine. In the early days of the HIV pandemic, newly diagnosed people measured time in months; now, the expected lifespan for people living with HIV has lengthened into years and decades. Antiretroviral therapy (ART) has saved millions of lives, and it took decades of research by many interdisciplinary teams to make this happen.
Despite this monumental success, there is still no cure. ART lowers viral burden, but it cannot clear the HIV reservoir—the pool of long-lived infected cells where HIV hides—which can not only persist but expand even in the presence of ART. As a result, people living with HIV have to take their medicine every day for the rest of their lives or risk viral rebound.
This is because there is currently no way to remove virus from infected cells and latent HIV is never truly silent. The viral genome, which is integrated into the host DNA in a form called the provirus, periodically reactivates in small “blips” triggered by events such as an immune response to another infection. Without ART, reactivated HIV infects and destroys the very cells that are needed to control HIV infection, leading to swift disease progression. With ART, these blips are quickly shut down, stopping the virus in its tracks and allowing the people living with HIV to live a long and healthy life.
Reactivation doesn’t have to be all bad, though. A cell making lots of viruses is no longer invisible to the immune system, meaning it can be eliminated by healthy immune cells. Theoretically, if all latently infected cells were awakened and eliminated while new infections were blocked by ART, the viral reservoir could be drained forever.
This “shock-and-kill” strategy is being pursued by researchers like Dr. Carley Gray, a postdoctoral fellow in the Emerman Lab. There’s an issue, though. There are drugs that can reactivate HIV—called latency reversal agents (LRAs)—but no drug reliably reactivates every infected cell.
The HIV provirus is subject to several host transcriptional blocks that prevent its reactivation, including blocks preventing transcription initiation and/or ending transcriptional elongation before a complete viral genome is produced. No drug on its own can overcome every block, nor are any targeted enough to specifically allow for HIV reactivation without off-target effects. Even combinations of multiple drugs haven’t been able to overcome this challenge.
“To further complicate things, cells derived from people living with HIV have more severe blocks to reactivation, i.e they are more difficult to reactivate than cell model systems,” Dr. Gray says. She emphasizes that this additional challenge “can cause promising drug combinations to fail, leaving researchers reeling for solutions.”
In a recent study in Elife, Dr. Gray and her colleagues sought to understand how HIV stays latent even in the presence of potent LRAs using a technique developed in their lab called HIV-CRISPR. The principle is simple: guide RNAs are tagged with a viral signal that ensures they are packaged into newly made virions, which are then sequenced. More virus produced means better reactivation, and more virus enriched with specific guide RNAs means that gene’s knockout helped reactivation. (See this article, this one, and this one too for more examples of how the Emerman lab has used HIV-CRISPR to understand HIV biology).
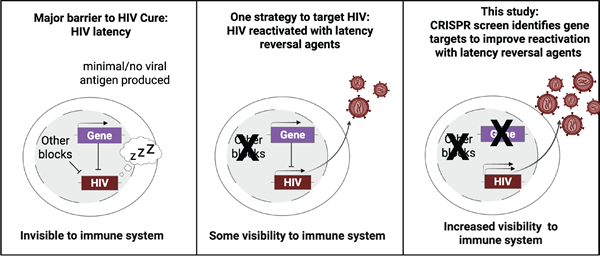
Overview of screening principle. Prior to latency reversal, latent HIV is blocked from reactivation by multiple mechanisms (left panel). Treatment with known latency reversal agents allows some HIV reactivation (middle panel). Increased HIV reactivation occurs when LRA treatment is combined with targeted knockout of genes that suppress HIV (right panel)
.Image provided by study authors.
Her top hit, a gene called INTS12, was enriched in both treated and untreated conditions, and, importantly, it popped up in two cell lines that have different sites of HIV integration. INTS12 encodes for the 12th subunit of the Integrator complex, which associates with RNA polymerase II to allow transcriptional elongation. (A quick note on RNA biology for those of us who don’t think about it that often: one way that we regulate gene expression is through deliberately pausing RNA polymerase II at the start of the transcription process. Various mechanisms, including the Integrator complex, determine whether and when RNA polymerase II continues down the gene or falls off).
On its own, knockout of INTS12 increased RNA polymerase II occupancy throughout the provirus region as well as transcription of the full-length HIV provirus. This suggests that INTS12 somehow blocks HIV transcript elongation and reactivation. Combined with either or both LRAs used in this study, the effect was even more pronounced.
“Importantly, these findings repeated when tested in cells from people living with HIV, a system where this particular LRA combination had previously failed,” Dr. Gray says. Additionally, “targeting INTS12 was found to have minimal effects on host genes, demonstrating that this may be a viable target for drug development.”
The authors wondered if the entire Integrator complex contributes to this HIV reactivation block. To interrogate this, they knocked out each member of the Integrator complex and evaluated viral reactivation. They already knew that knockout of INTS12, which helps Integrator read chromatin, allowed HIV to reactivate, and they found that all members of the RNA cleavage and phosphatase modules also appear to be crucial to keeping HIV repressed.
Another key finding was that targeting INTS12 could improve HIV reactivation when cells were treated with other LRAs that work by different mechanisms than the combination used in the initial screen. The synergy with many different LRAs “makes sense when you understand that many LRAs act prior to the Integrator’s elongation effects” explains Dr. Gray. She hypothesizes that the Integrator complex may pose a general block to HIV reactivation, and that INTS12, which appears to be particularly specific for HIV, may be a critical barrier.
“While this is a great step in the right direction, there is much to optimize for shock-and-kill,” says Dr. Gray. “But fortunately, there are teams of scientists from across the world doing just that. In the future, it will be the combined efforts of many scientists that may make this a viable approach to cure HIV.”
Fred Hutch/University of Washington/Seattle Children’s Cancer Consortium Member Dr. Michael Emerman contributed to this research.
The spotlighted research was funded by the National Institutes of Allergy and Infectious Disease, the National Institute on Drug Abuse, the National Cancer Institute, the Hartwell Foundation, and the National Cancer Institute.
Gray CN, Ashokkumar M, Janssens DH, Kirchherr JL, Allard B, Hsieh E, Hafer TL, Archin NM, Browne EP, Emerman M. 2025. Integrator complex subunit 12 knockout overcomes a transcriptional block to HIV latency reversal. eLife 13:RP103064
Hannah Lewis is a postdoctoral research fellow with Jim Boonyaratanakornkit’s group in the Vaccine and Infectious Disease Division (VIDD). She is developing screens to find rare B cells that produce protective antibodies against human herpesviruses. She obtained her PhD in molecular and cellular biology from the University of Washington.
Fred Hutchinson Cancer Center
Fred Hutchinson Cancer Center unites innovative research and compassionate care to prevent and eliminate cancer and infectious disease. We’re driven by the urgency of our patients, the hope of our community and our passion for discovery to pursue scientific breakthroughs and healthier lives for every person in every community.
Our values are grounded in and expressed through the principles of diversity, equity and inclusion (DEI). Our mission is directly tied to the humanity, dignity and inherent value of each employee, patient, community member and supporter. Our actions are driven by the commitments expressed in our values: Collaboration, Compassion, Determination, Excellence, Innovation, Integrity and Respect.
Fred Hutch is an independent, nonprofit organization, that also serves as UW Medicine’s cancer program. This unique relationship allows for enhanced care coordination with one of the world's leading integrated health systems .www.fredhutch.org
Contact:
Claire Hudson
She/Her/Hers
Senior Communications Manager
Marketing & Communications
Fred Hutchinson Cancer Center
M 206.919.8300
crhudson@fredhutch.org
Source: Fred Hutch Cancer Centerhttps://www.fredhutch.org/en/news/spotlight/2025/05/hb-gray-elife.html
"Reproduced with permission - Fred Hutchinson Cancer Center"
Fred Hutchinson Cancer Center
Back to ...
Positively Positive - Living with HIV/AIDS:
HIV/AIDS News
For more HIV and AIDS News visit...
Positively Positive - Living with HIV/AIDS: HIV/AIDS News Archive
|