
|
The Findings Advance AIDS Vaccine Development
 LA JOLLA, CA, October 13, 2011 - Researchers at The Scripps Research Institute have
uncovered the surprising details of how a powerful anti-HIV antibody grabs hold of the virus. The findings, published in Science Express
on October 13, 2011, highlight a major vulnerability of HIV and suggest a new target for vaccine development. LA JOLLA, CA, October 13, 2011 - Researchers at The Scripps Research Institute have
uncovered the surprising details of how a powerful anti-HIV antibody grabs hold of the virus. The findings, published in Science Express
on October 13, 2011, highlight a major vulnerability of HIV and suggest a new target for vaccine development.
"What's unexpected and unique about this antibody is that it not only attaches to the sugar coating of the virus but also reaches through
to grab part of the virus's envelope protein," said the report's co-senior author Dennis Burton, a professor at The Scripps Research
Institute and scientific director of the International AIDS Vaccine Initiative's (IAVI) Neutralizing Antibody Center, based on the
Scripps Research La Jolla campus.
"We can now start to think about constructing mimics of these viral structures to use in candidate vaccines," said co-senior author Ian
Wilson, who is Hansen Professor of Structural Biology and member of the Skaggs Institute for Chemical Biology at Scripps Research.
Other institutions in the United States, United Kingdom, Japan, and the Netherlands contributed to the research as part of an ongoing global
HIV vaccine development effort.
"The study's results open the door to a whole new approach to drug design against HIV protease," said Scripps Research Associate Professor
C. David Stout, senior author of the study. "The fragments
bound at not one, but two, different crevices in protease outside the active site. This is an important proof-of-concept that the protease
molecule has two non-active site binding pockets ('allosteric sites')
which can now be exploited as a powerful new strategy to combat drug-resistance in HIV."
Getting a Better Grip on HIV
Researchers from the current team recently isolated the new antibody and 16 others from the blood of HIV-infected volunteers, in work they
reported online in the journal Nature on August 17, 2011. Since the 1990s, Burton, Wilson, and other researchers have been searching for
such "broadly neutralizing" antibodies against HIV-antibodies that work against many of the various strains of the fast-mutating
virus-and by now have found more than a dozen. PGT 128, the antibody described in the new report, can neutralize about
70 percent of globally circulating HIV strains by blocking their ability to infect cells. It also can do so much
more potently-in other words, in smaller concentrations of antibody molecules-than any previously reported
broadly neutralizing anti-HIV antibody.
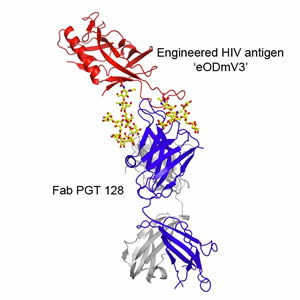
The new report illuminates why PGT 128 is so effective at neutralizing HIV. Using the Wilson lab's expertise in X-ray crystallography,
Robert Pejchal, a research associate in the Wilson lab, determined the structure of PGT 128 joined to its binding site on molecular
mockups of the virus, designed in part by Robyn Stanfield and Pejchal in the Wilson group and Bill Schief, now an IAVI principal
scientist and associate professor at Scripps Research, and his group. With these structural data, and by experimentally mutating
and altering the viral target site, they could see that PGT 128 works in part by binding to glycans on the viral surface. .
Thickets of these sugars normally surround HIV's envelope protein, gp120, largely shielding it from attack by the immune system.
Nevertheless, PGT 128 manages to bind to two closely spaced glycans, and at the same time reaches through the rest of the "glycan
shield" to take hold of a small part of structure on gp120 known as the V3 loop. This penetration of the glycan shield by PGT 128
was also visualized by electron microscopy with a trimeric form of the gp120/gp41 envelope protein of HIV-1 by Reza Kayat and
Andrew Ward of Scripps Research; this revealed that the PGT 128 epitope appears to be readily accessible on the virus.
"Both of these glycans appear in most HIV strains, which helps explain why PGT 128 is so broadly neutralizing," said Katie J. Doores, a
research associate in the Burton lab who was one of the report's lead authors. PGT 128 also engages V3 by its backbone structure, which
doesn't vary as much as other parts of the virus because it is required for infection.
PGT 128's extreme potency is harder to explain. The antibody binds to gp120 in a way that presumably disrupts its ability to lock onto
human cells and infect them. Yet it doesn't bind to gp120 many times more tightly than other anti-HIV antibodies. The team's analysis
hints that PGT 128 may be extraordinarily potent because it also binds two separate gp120 molecules, thus tying up not one but two
cell-infecting structures. Other mechanisms may also be at work.
Toward an AIDS Vaccine
Researchers hope to use the knowledge of these antibodies' binding sites on HIV to develop vaccines that stimulate a long-term-perhaps
lifetime-protective antibody response against those same vulnerable sites.
"We'll probably need multiple targets on the virus for a successful vaccine, but certainly PGT 128 shows us a very good target,"
said Burton.
Intriguingly, the basic motif of PGT 128's target may mark a general vulnerability for HIV. "Other research is also starting to
suggest that you can grab onto two glycans and a beta strand and get very potent and broad neutralizing antibodies against HIV,"
Wilson said.
In addition to Pejchal, Doores, and Khayat, Laura M. Walker of Scripps Research and Po-Ssu Huang of University of Washington at Seattle
were co-first authors of the study, "A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield." Along
with Wilson, Burton, and Ward, additional contributors were Sheng-Kai Wang, Chi-Huey Wong, Robyn L. Stanfield, Jean-Philippe Julien,
Alejandra Ramos, Ryan McBride, and James C. Paulson of Scripps Research; William R. Schief of Scripps Research, IAVI, and
University of Washington at Seattle; Pascal Poignard of IAVI; Max Crispin and Christopher N. Scanlan of the University
of Oxford; Rafael Depetris and John P. Moore of Weill Medical College of Cornell University; Umesh Katpally,
Andre Marozsan, Albert Cupo, and William C. Olson of Progenics Pharmaceuticals; Sebastien Maloveste of
the National Institute of Allergy and Infectious Diseases at the National Institutes of Health;
Yan Liu and Ten Feizi of Imperial College, London; Yukishige Ito of the RIKEN Advanced Science
Institute in Japan; and Cassandra Ogohara of University of Washington at Seattle. Portions
of this research were carried out at the Stanford Synchotron Radiation Lightsource.
Diffraction data were also collected at the GM/CA-CAT beamline at the Argonne National Laboratory.
The research was supported by the International AIDS Vaccine Initiative, National Institutes of Health, the U.S. Department of Energy, the
Canadian Institutes of Health Research, the UK Research Councils, the Ragon Institute, and other organizations.
###
About The Scripps Research Institute
The Scripps Research Institute is one of the world's largest
independent, non-profit biomedical research organizations. Scripps Research is internationally recognized for its discoveries in
immunology, molecular and cellular biology, chemistry, neuroscience, and vaccine development, as well as for its insights into
autoimmune, cardiovascular, and infectious disease. Headquartered in La Jolla, California, the institute also includes a
campus in Jupiter, Florida, where scientists focus on drug discovery and technology development in addition to basic
biomedical science. Scripps Research currently employs about 3,000 scientists, staff, postdoctoral fellows, and
graduate students on its two campuses. The institute's graduate program, which awards Ph.D. degrees in biology
and chemistry, is ranked among the top ten such programs in the nation. For more information,
see www.scripps.edu .
For information:
Mika Ono
Tel: 858-784-2052
Fax: 858-784-8136
mikaono@scripps.edu
Source: The Scripps Research Institute
http://www.scripps.edu/news/press_releases/20111013burton.html
|